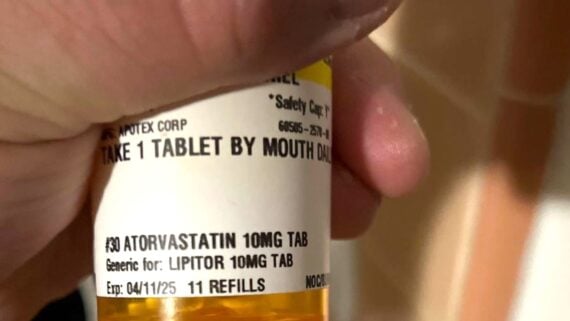
The FDA has probably never been busier, and while they’re monitoring food for both humans and pets alike, they’re also on top of prescription medication. Most recently, they’ve swatted more than 140,000 bottles of a prescription cholesterol medication. Here’s what to know about the cholesterol medication recall.
What Medication Is Being Recalled?
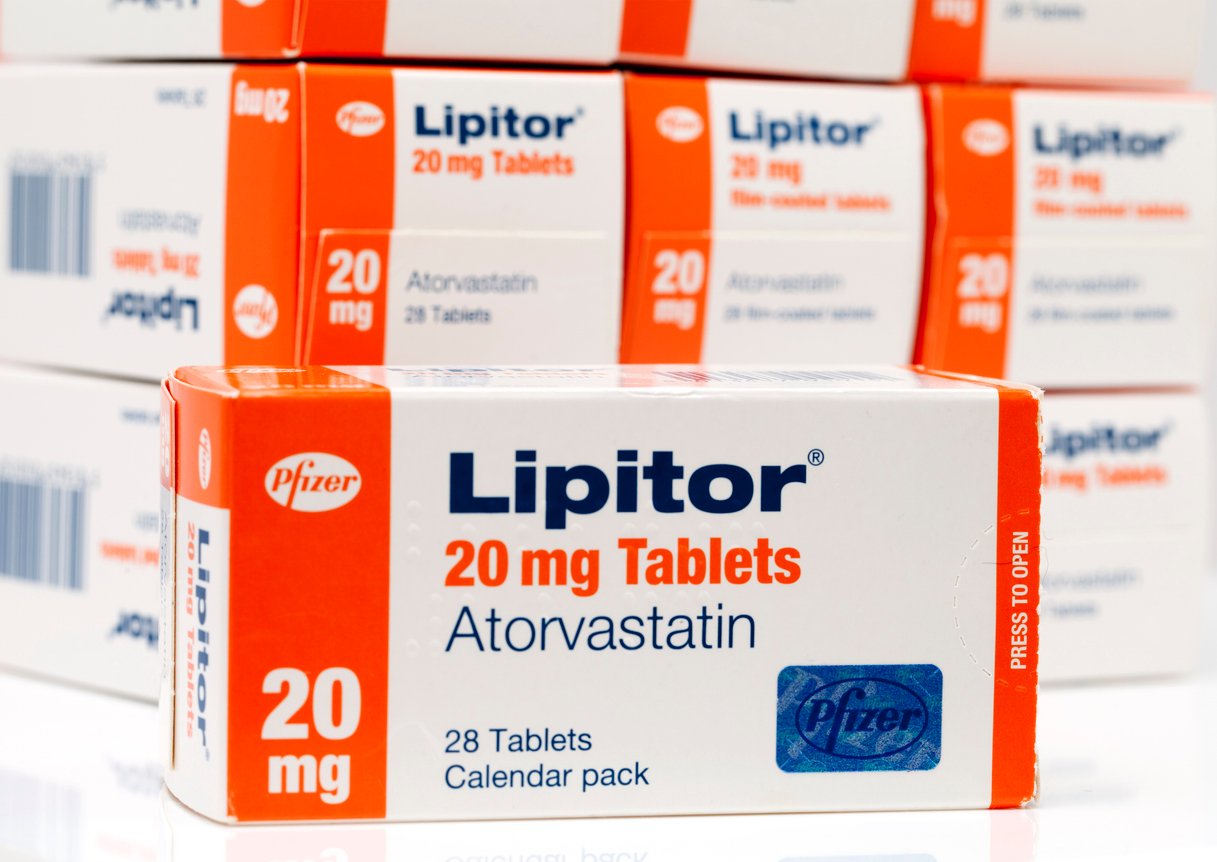
The recalled drug is Atorvastatin Calcium, a statin. Statins are a class of medications used to lower cholesterol and prevent and reduce heart disease. Atorvastatin Calcium is a generic version of Lipitor.
Why Is the Drug Being Recalled?

The pills are being recalled due to failed dissolution specifications, according to the FDA, which means they don’t dissolve properly. This means the drug has a much higher percentage of not working correctly, or worse. According to the FDA’s risk classification, this could “cause temporary or medically reversible adverse health consequences.”
Who Manufactured the Drug?

The drugs were manufactured by Alkem Laboratories of India, and distributed in 90-count, 500-count, and 1,000-count bottles..
Which Prescription Bottles Are Being Recalled?
These are the prescription medications being recalled:
Atorvastatin Calcium Tablets USP, 10 mg, 90-count bottle (NDC 67877-511-90)
- Lot#: 25141249, Exp. Feb. 2027
- Lot#: 24144938, Exp. Nov. 2026
- Lot#: 24144868, Exp. Nov. 2026
- Lot#: 24144867, Exp. Nov. 2026
- Lot#: 24144458, Exp. Sep. 2026
- Lot#: 24143994, Exp. Sep. 2026
- Lot#: 24142987, Exp. July 2026
- Lot#: 24143316, Exp. July 2026
Atorvastatin Calcium Tablets USP, 10 mg, 500-count bottle (NDC 67877-511-05)
- Lot#: 25141249, Exp. Feb. 2027
- Lot#: 24144938, Exp. Nov. 2026
- Lot#: 24144868, Exp. Nov. 2026
- Lot#: 24144867, Exp. Nov. 2026
- Lot#: 24144458, Exp. Sep. 2026
- Lot#: 24143994, Exp. Sep. 2026
- Lot#: 24142987, Exp. July 2026
- Lot#: 24143316, Exp. July 2026
Atorvastatin Calcium Tablets USP, 10 mg, 1000-count bottle (NDC 67877-511-10)
- Lot#: 25141249, Exp. Feb. 2027
- Lot#: 24144938, Exp. Nov. 2026
- Lot#: 24144868, Exp. Nov. 2026
- Lot#: 24144867, Exp. Nov. 2026
- Lot#: 24144458, Exp. Sep. 2026
- Lot#: 24143994, Exp. Sep. 2026
- Lot#: 24142987, Exp. July 2026
- Lot#: 24143316, Exp. July 2026
Atorvastatin Calcium Tablets USP, 40 mg, Rx Only, 90-count bottle (NDC 67877-513-90)
- Lot#: 25140933, Exp. Feb. 2027
- Lot#: 25140477, Exp. Dec. 2026
- Lot#: 24144254, Exp. Oct. 2026
- Lot#: 24144163, Exp. Sep. 2026
- Lot#: 24143995, Exp. Sep. 2026
Atorvastatin Calcium Tablets USP, 40 mg, Rx Only, 500-count bottle (NDC 67877-513-05)
- Lot#: 25140933, Exp. Feb. 2027
- Lot#: 25140477, Exp. Dec. 2026
- Lot#: 24144254, Exp. Oct. 2026
- Lot#: 24144163, Exp. Sep. 2026
- Lot#: 24143995, Exp. Sep. 2026
Atorvastatin Calcium Tablets USP, 40 mg, Rx Only, 1000-count bottle (NDC 67877-513-10)
- Lot#: 25140933, Exp. Feb. 2027
- Lot#: 25140477, Exp. Dec. 2026
- Lot#: 24144254, Exp. Oct. 2026
- Lot#: 24144163, Exp. Sep. 2026
- Lot#: 24143995, Exp. Sep. 2026
Atorvastatin Calcium Tablets USP, 20 mg, Rx Only, 90-count bottle (TK)
- Lot #: 25140150, Exp. Dec. 2026
- Lot #: 25140173, Exp. Dec. 2026
- Lot #: 25140172, Exp. Dec. 2026
- Lot #: 24144720, Exp. Nov. 2026
- Lot #: 24144798, Exp. Nov. 2026
- Lot #: 24144692, Exp. Oct. 2026
- Lot #: 24143755, Exp. Aug. 2026
- Lot #: 24143913, Exp. Aug. 2026
- Lot #: 24143754, Exp. Aug. 2026
- Lot #: 24143047, Exp. June 2026
- Lot #: 24142936, Exp. July 2026
Atorvastatin Calcium Tablets USP, 20 mg, Rx Only, 500-count bottle (NDC 67877-512-05)
- Lot #: 25140150, Exp. Dec. 2026
- Lot #: 25140173, Exp. Dec. 2026
- Lot #: 25140172, Exp. Dec. 2026
- Lot #: 24144720, Exp. Nov. 2026
- Lot #: 24144798, Exp. Nov. 2026
- Lot #: 24144692, Exp. Oct. 2026
- Lot #: 24143755, Exp. Aug. 2026
- Lot #: 24143913, Exp. Aug. 2026
- Lot #: 24143754, Exp. Aug. 2026
- Lot #: 24143047, Exp. June 2026
- Lot #: 24142936, Exp. July 2026
Atorvastatin Calcium Tablets USP, 20 mg, Rx Only, 1000-count bottle (NDC 67877-512-10)
- Lot #: 25140150, Exp. Dec. 2026
- Lot #: 25140173, Exp. Dec. 2026
- Lot #: 25140172, Exp. Dec. 2026
- Lot #: 24144720, Exp. Nov. 2026
- Lot #: 24144798, Exp. Nov. 2026
- Lot #: 24144692, Exp. Oct. 2026
- Lot #: 24143755, Exp. Aug. 2026
- Lot #: 24143913, Exp. Aug. 2026
- Lot #: 24143754, Exp. Aug. 2026
- Lot #: 24143047, Exp. June 2026
- Lot #: 24142936, Exp. July 2026
Atorvastatin Calcium Tablets USP, 80 mg, Rx Only, 90-count bottle (NDC 67877-514-90)
- Lot#: 25140249, Exp. Dec. 2026
- Lot#: 25140247, Exp. Dec. 2026
- Lot#: 24144999, Exp. Nov. 2026
- Lot#: 24144942, Exp. Nov. 2026
- Lot#: 24144845, Exp. Nov. 2026
- Lot#: 24144713, Exp. Nov. 2026
- Lot#: 24144652, Exp. Oct. 2026
- Lot#: 24143898, Exp. Aug. 2026
- Lot#: 24143412, Exp. Aug. 2026
- Lot#: 24143582, Exp. Aug. 2026
Atorvastatin Calcium Tablets USP, 80 mg, Rx Only, 500-count bottle (TK)
- Lot#: 25140249, Exp. Dec. 2026
- Lot#: 25140247, Exp. Dec. 2026
- Lot#: 24144999, Exp. Nov. 2026
- Lot#: 24144942, Exp. Nov. 2026
- Lot#: 24144845, Exp. Nov. 2026
- Lot#: 24144713, Exp. Nov. 2026
- Lot#: 24144652, Exp. Oct. 2026
- Lot#: 24143898, Exp. Aug. 2026
- Lot#: 24143412, Exp. Aug. 2026
- Lot#: 24143582, Exp. Aug. 2026
What Should I Do If I Have the Recalled Medication?

Neither the FDA nor the manufacturer has released specific guidance on the recalled medications. If a recall involves prescription medication, WebMD advises patients to call their doctor or pharmacist as soon as possible to determine what replacement is needed and how best to dispose of the recalled medication safely.
Trending on Cheapism
More Recall News From Cheapism

- Thousands of Coca-Cola Cans Recalled Due to Potential Metal Contamination — The recall involves three different Coca-Cola products.
- Dog and Cat Food From 3 Major Brands Recalled Over Salmonella — Pet owners will want to check the pantry as the recall involves several different products from multiple brands.
- More Frozen Shrimp Recalled Over Possible Radioactive Contamination — The frozen shrimp recall has expanded to include more retailers.