If Kirkland Signature Severe Cold & Flu Plus Congestion medicine is sitting in your medicine cabinet, it’s time to toss it. Costco has yanked it off the shelves due to potential foreign material contamination and advises customers do the same.
Here is everything you need to know about the recall.
What Does Costco Say About the Cold Medicine Recall?
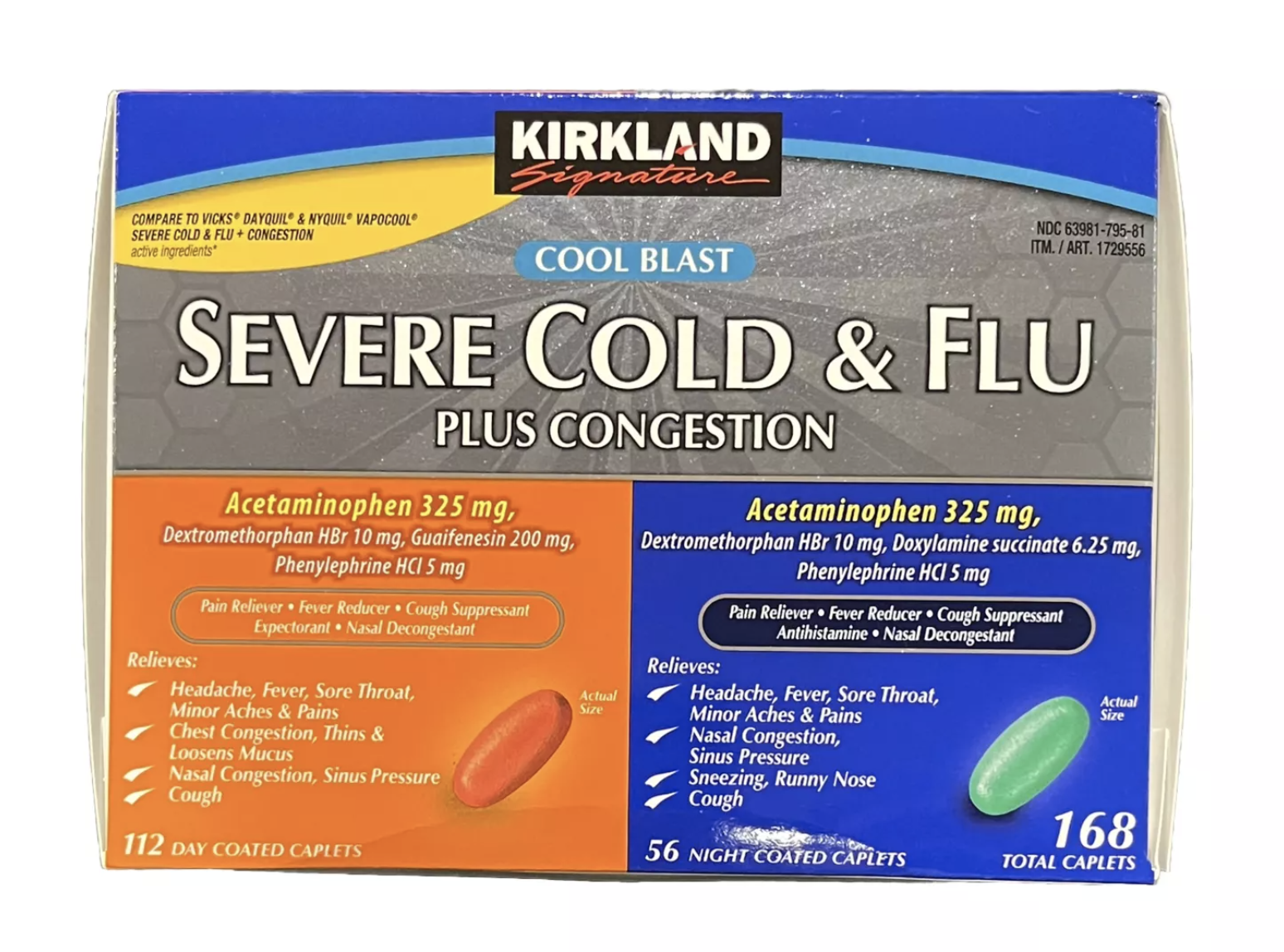
On January 2, the wholesale giant notified its members in a statement about the voluntary recall of Kirkland Signature Severe Cold & Flu Plus. “Out of an abundance of caution, LNK has initiated a recall for the accidental release and shipment of a specific lot code that was rejected due to potential foreign material contamination,” reads the statement.
Is Your Cold Medicine Affected?
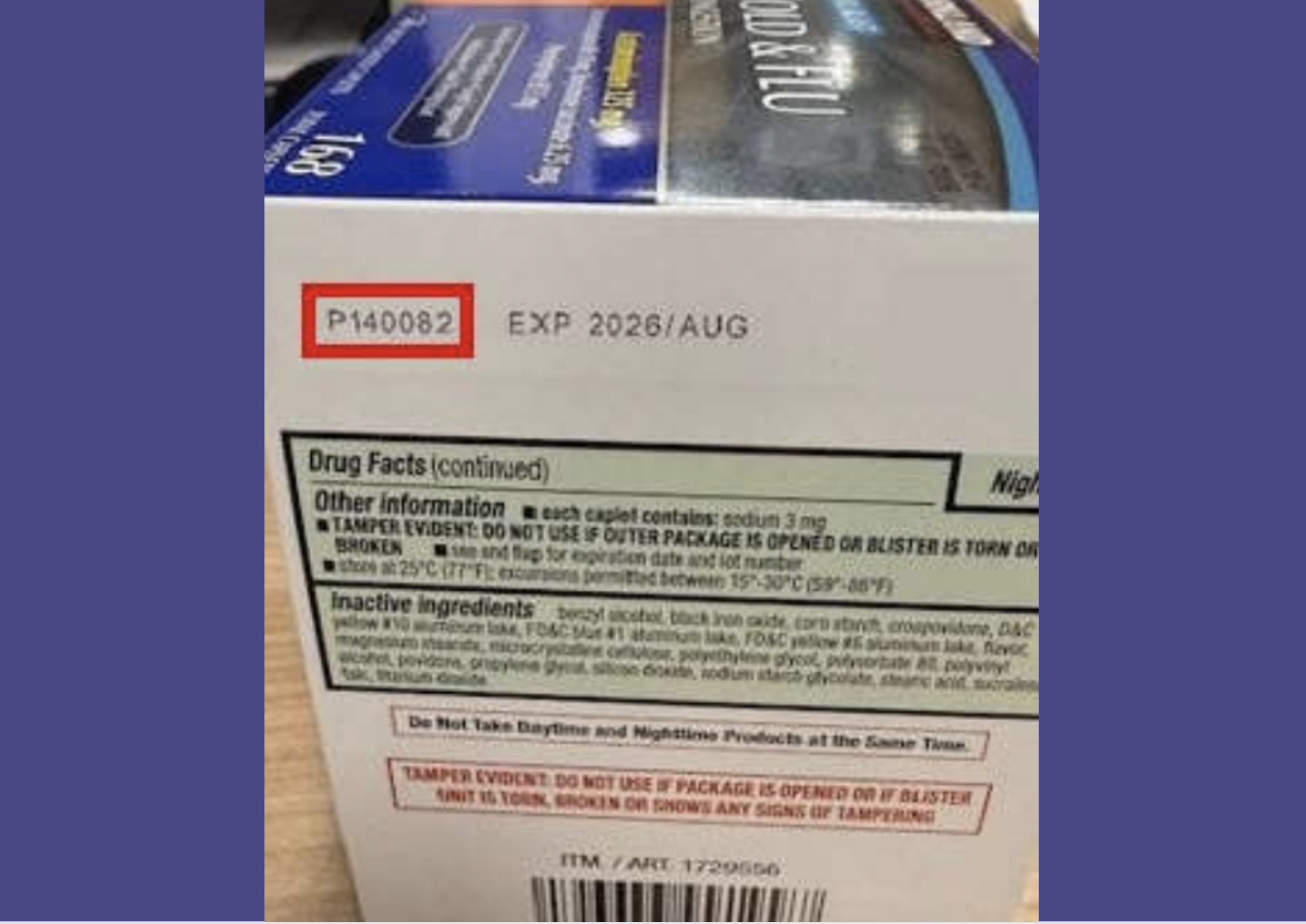
To see if you’re affected, look for item number 1729556 and lot code P140082 on the packaging. If you find it, don’t use it and return it to Costco for a full refund. The affected lot was sold between October 30 and November 30, 2024, in Midwest and Southeast stores.
Questions or Concerns About the Costco Recall?

In case you have any concerns about the affected medicine Costco advises to call LNK International Inc. at (800) 426-9391 or email [email protected], if they have any issues or concerns.
Health Officials Warn of ‘Quad-demic’ Amid Recall
This recall comes at a lousy time, with health officials warning of a “quad-demic” — a surge in COVID-19, flu, RSV, and adenoviruses. So, while ditching the bad meds, maybe stock up on some hand sanitizer, chicken soup, and remedies that haven’t been recalled.
For more Costco news, sign up for our free newsletters.




